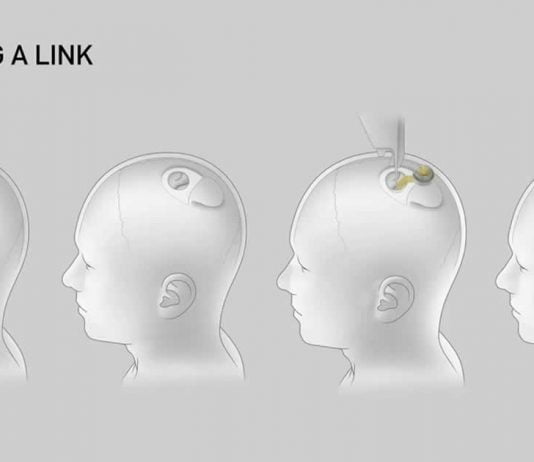
After months of anticipation, Neuralink, the brain implant startup led by Elon Musk, has received approval from the US Food and Drug Administration (FDA) to initiate human trials. The announcement comes almost six months after Musk shared the latest information on Neuralink, generating significant buzz and speculation about the company’s progress.
While Neuralink has yet to begin recruiting subjects for the trials, specific details regarding the trial parameters remain undisclosed. Typically, initial trials focus on ensuring safety rather than measuring efficacy and involve a small number of participants. Neuralink’s ambitious goals require extensive testing, as the company is developing both a brain implant and a surgical robot to perform the implantation procedure.
Although human trials for various brain implants have been undertaken in the past, Neuralink’s concentration on the surgical robot distinguishes it. By merging cutting-edge implant technology with modern robotics, the company hopes to revolutionise the industry, opening the door for more accurate and efficient treatments.
Neuralink has recently experienced hurdles and disagreements with federal authorities, making FDA clearance a key milestone for the firm. When the FDA denies a clinical study application, it often offers extensive reasons for its decision, outlining any problems regarding the experiment’s design. As a result, Neuralink’s ability to handle these regulatory concerns in a very short amount of time is seen positively.
The approval of human trials is a crucial step forward for Neuralink as it approaches its ultimate goal of developing a brain-computer interface that might possibly revolutionise how people interact with technology. Despite the uncertainties surrounding the next trials, this breakthrough gives Neuralink’s personnel and its visionary CEO, Elon Musk, hope.
As Neuralink continues its ground-breaking research, the results of the human trials will definitely pique people’s attention and determine the future of brain-computer interfaces.